Lewis Dot Structure For Oxygen Atom slidesharedocs

70 More Lewis Dot Structures. Element number 8 and a member of the Chalcogen Family or Group 16 of the periodic table.. Ozone is an allotrope of oxygen, and is much less stable. Ultraviolet light cause it to decompose in our ozone layer, therefore it shielding people below it.
Lewis Dot Structure for O3 (Ozone) YouTube

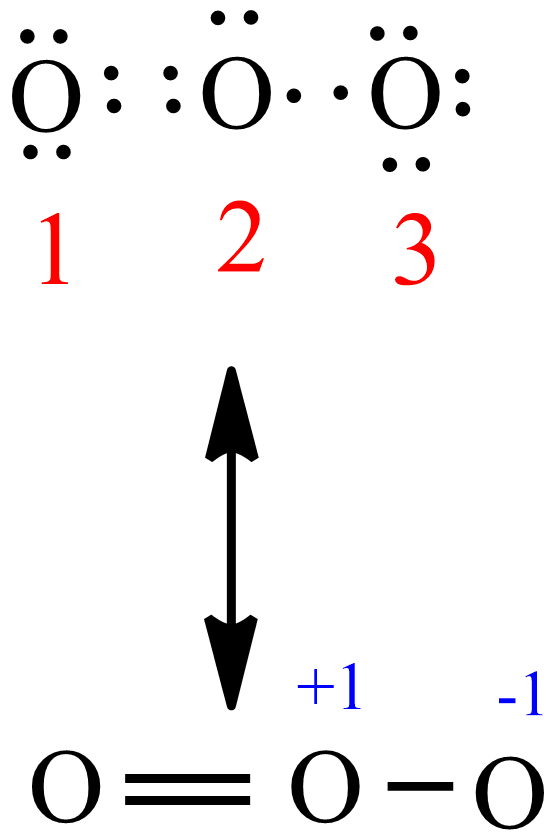
The typical Lewis structure of ozone depicts formal charge separation Simple VESPER requires that we distribute 3xx6=18 "valence electrons" across 3 centres: O=O^(+)-O^(-) From the left, O_1, has TWO lone pairs; O_2 has ONE lone pairs; and O_3 has THREE lone pairs. And thus the formal charge of each oxygen atom (8e^-,7e^-,9e^-) is 0,+1, -1 respectively.
O3 Resonance Structures (Ozone) YouTube

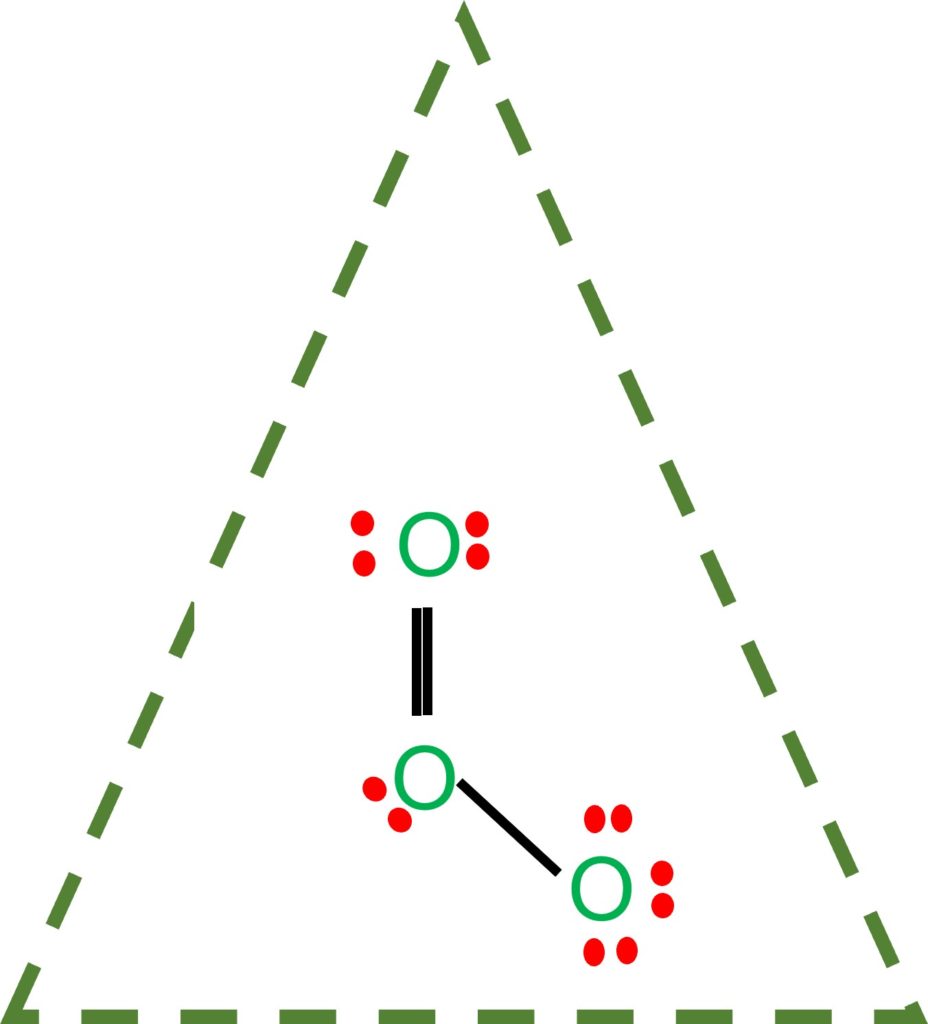
Equivalent Lewis dot structures, such as those of ozone, are called resonance structures. The position of the atoms is the same in the various resonance structures of a compound, but the position of the electrons is different. Double-headed arrows link the different resonance structures of a compound:
Calculating O3 Formal Charges Calculating Formal Charges for O3 (Ozone) YouTube

This chemistry video tutorial explains how to draw the lewis structure of O3. It also discusses the molecular geometry, bond angle, hybridization, and forma.
The Lewis dot structure for the ozone molecule is
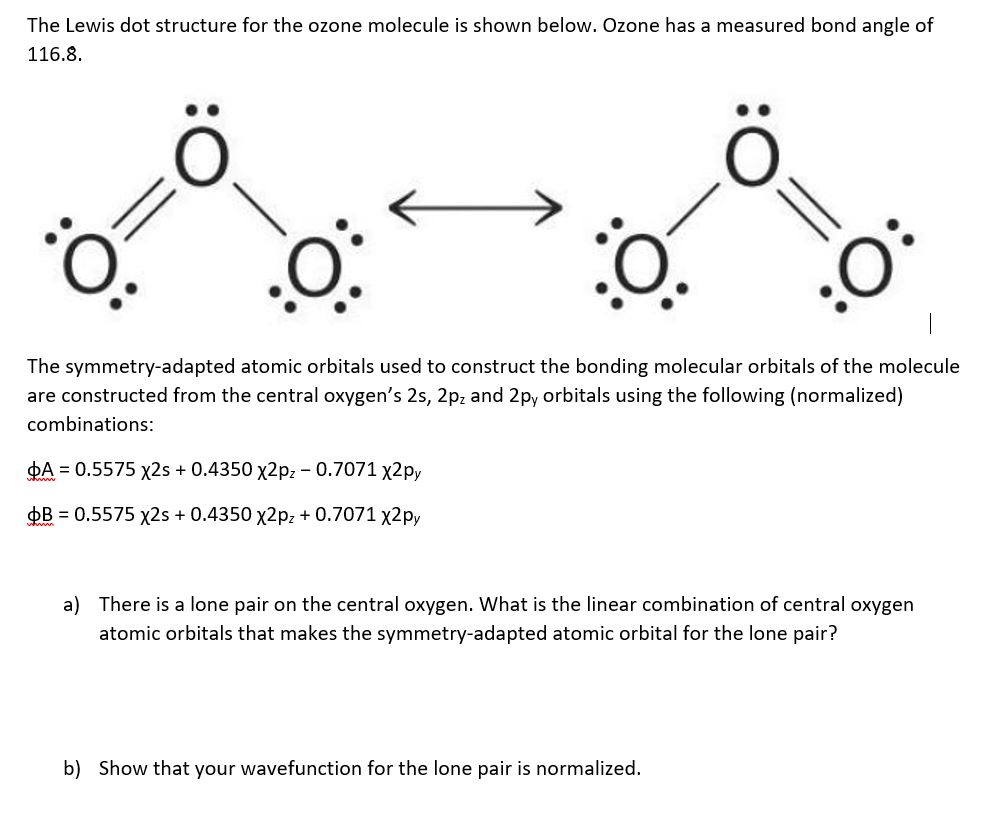
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, and.
Find the formal charge of ‘O’ in ozone.
Ozone is one of the most common examples used to study the Lewis structure. The molecule of Ozone has three oxygen atoms. It is written as O3 in the core chemistry equations. To understand the hybridization, polarity and molecular geometry of the Ozone molecule it is crucial to know the Lewis structure of the same. Name of molecule.
SOLVED Draw the Lewis structure of ozone (O3) and then determine its molecular geometry.
I quickly take you through how to draw the Lewis Structure of O3 (Ozone). I also go over the resonance, hybridization, shape and bond angle.
Ozone Molecule Lewis Structure

Lets start with a look at the Lewis Dot Structure of Ozone. Illustration of Resonance: Ozone. Ozone (O 3) is an allotrope of oxygen, with diatomic oxygen (O 2) being the most common form of oxygen. Ozone is a very reactive form of oxygen that has detrimental health effects (which is why ozone alerts are posted along the highways of cities), but.
O3 Lewis Structure Step By Step Drawing What's Insight
A step-by-step explanation of how to draw the O3 Lewis Dot Structure (Ozone).For the O3 structure use the periodic table to find the total number of valence.
Ozone Molecule Lewis Structure

Ozone is an allotropic molecular form of oxygen containing three atoms of oxygen (O3). Ozone O3 is generated through the passage of oxygen O 2 through a high voltage potential resulting in the attachment and formation of a third oxygen atom. The molecular formula for ozone (O 3) was established by Soret (1863) by taking the ratio of the change.
O3 Lewis Structure (Ozone) Lewis, Ozone, Chemistry

A step-by-step explanation of how to draw the O3 Lewis Dot Structure (Ozone).For the O3 structure use the periodic table to find the total number of valence.
O3 Lewis Structure Formal Charge Basics of Chemistry

Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
Lewis Dot Structure (Ozone) Science ShowMe

The Lewis Structure of Ozone has:* three oxygen atoms in a row. It is not a ring, although that might be tempting.* two resonance structures* a lone pair on.
Ozone Lewis Structure Resonance

In this post, we will be drawing the Lewis structure of ozone, O 3.. The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level.For example: Na - 1s 2 2s 2 2p 6 3s 1, Cl - 1s 2 2s 2 2p 6 3s 2 3p 5 The number of valence electrons, for main group.
Ozone Molecule Lewis Structure
A step-by-step explanation of how to draw the O3 Lewis Dot Structure (Ozone).For the O3 structure use the periodic table to find the total number of valence.
Ozone Molecule Lewis Structure

Lewis Structure of O3. Here, we will be dealing with ozone, the molecular formula is O3. The below discussion, therefore, will be based on finding out the Lewis Structure of O3. Ozone consists of three oxygen atoms. Oxygen belongs to group VI of the periodic table with an atomic no of 8. It thus has 6 valence electrons.